Antibiotic Treatment Response in Chronic Lyme Disease: Why Do Some Patients Improve While Others Do Not?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Study Participants
2.2.1. Treatment Response Subgroups
2.2.2. Demographics
2.3. Methodology Overview
- Treatment approach,
- Treatment duration, and
- Treatment focus of clinician overseeing care.
3. Results
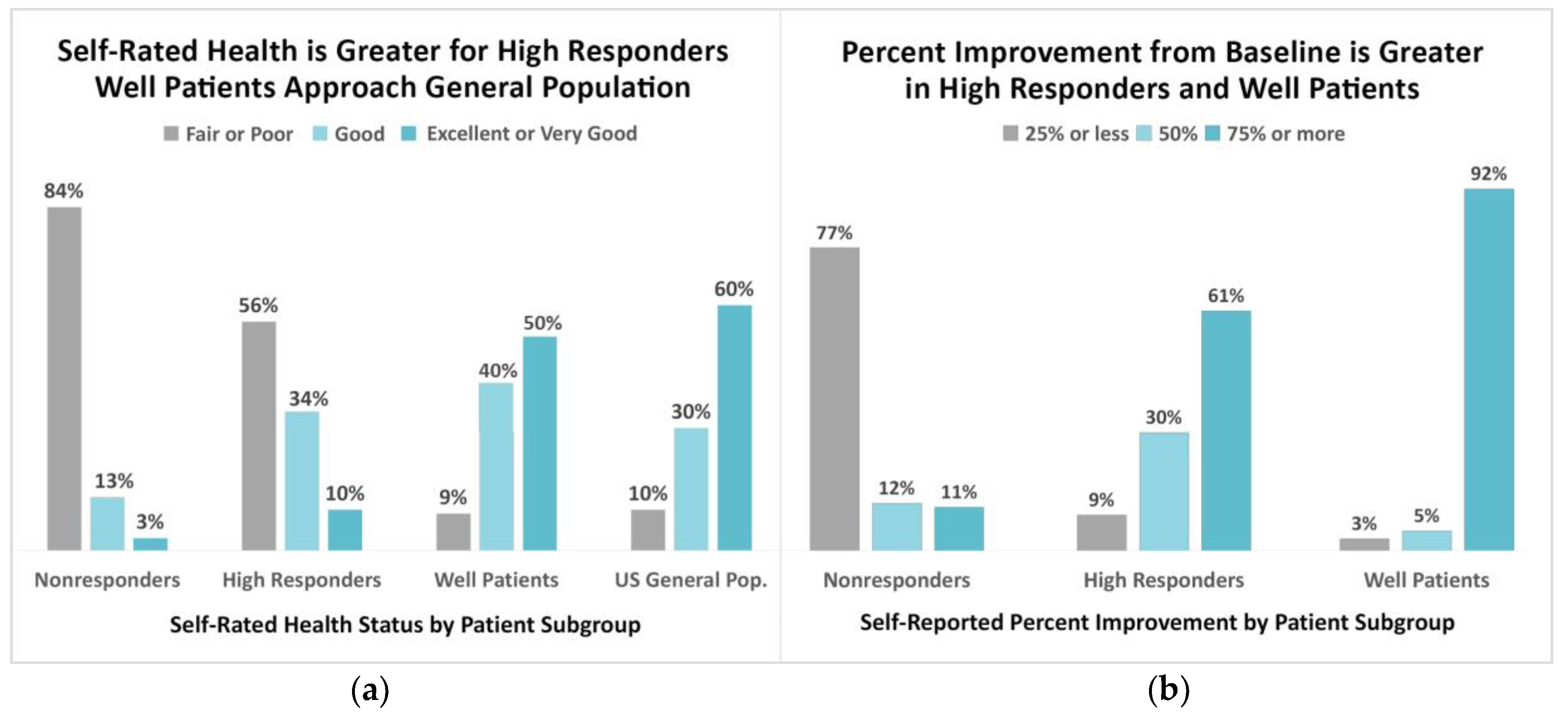
3.1. Indicators of Wellness among Patient Subgroups
3.2. Predictive Features Associated with Improved Outcomes
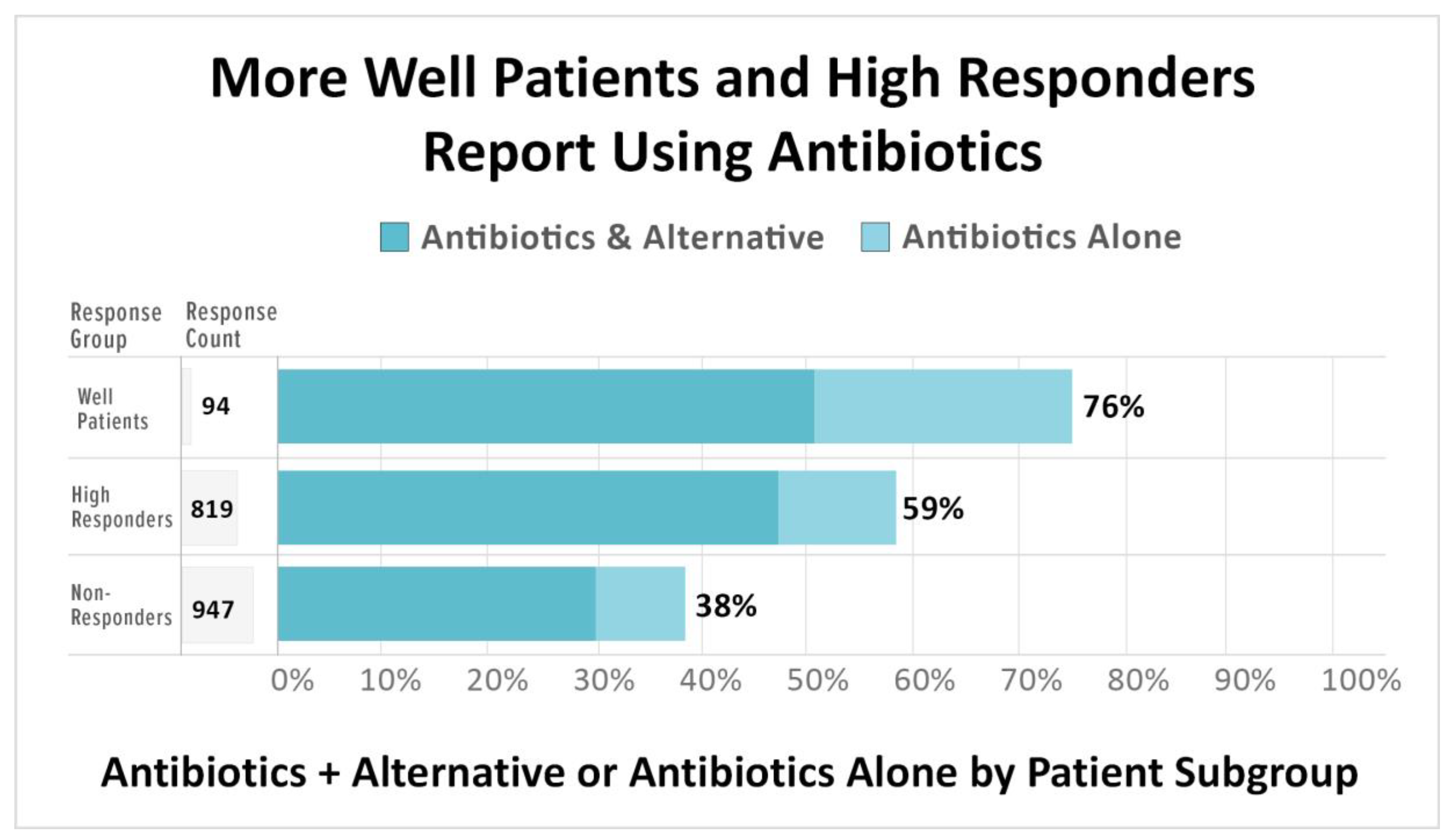
3.2.1. The Use of Antibiotics is Associated with High Treatment Response and Wellness
3.2.2. Longer Treatment Durations are Associated with High Treatment Response and Wellness
3.2.3. Clinical Oversight by a Clinician Whose Practice Focuses on Tick-Borne Diseases Is Associated with Higher Treatment Response and Wellness
4. Discussion
4.1. The MyLymeData Patient Registry
4.2. Patient-Reported Indicators of Health Status
4.2.1. Self-Rated Health Status
4.2.2. Self-Rated Percentage of Improvement
4.2.3. Symptom Severity
4.3. Features Associated with Improved Treatment Outcomes
4.3.1. The Use of Antibiotics Is Associated with Improved Treatment Outcomes
4.3.2. Longer Treatment Durations Associated with Greater Treatment Response
4.3.3. Clinical Focus of Treating Practitioner
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GROC | Global Rating of Change |
| LLMD | Lyme-literate medical doctor |
| CLD | chronic Lyme disease |
| AHRQ | Agency for Healthcare Research and Quality |
| BRFSS | Behavioral Risk Factor Surveillance System |
| SRHS | Self-Rated Health Status |
| SF-36 | 36-Item Short Form Survey |
| AB | Antibiotics |
| ALT | alternative treatments |
| PRO | patient-reported outcome |
| CDC | Centers for Disease Control and Prevention |
| IDSA | Infectious Diseases Society of America |
| ILADS | International Lyme & Associated Diseases Society |
Appendix A. The Global Rating of Change Scale
- Nonresponders, who answered between −7 and 0, indicating that there was no improvement.
- Low responders, who answered between 1 and 3, indicating that there was slight improvement.
- High responders, who answered between 4 and 7, indicating that there was substantial improvement.
- Worse—→How Much Worse
- Better—→How Much Better
- Unchanged includes “Almost the same” for both Worse and Better responses
Appendix B. Common Symptoms of Chronic Lyme Disease
- Fatigue
- Headache
- Joint Pain
- Muscle aches
- Neuropathy
- Twitching
- Memory Loss
- Cognitive Impairment
- Sleep Impairment
- Psychiatric
- Heart related
- Gastrointestinal
References
- Centers for Disease Control and Prevention. CDC Provides Estimate of Americans Diagnosed with Lyme Disease Each Year. Available online: http://www.cdc.gov/media/releases/2013/p0819-lyme-disease.html (accessed on 30 September 2020).
- Aucott, J.N.; Rebman, A.W.; Crowder, L.A.; Kortte, K.B. Post-treatment Lyme disease syndrome symptomatology and the impact on life functioning: Is there something here? Qual. Life Res. 2013, 22, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, A.G.; Herman, R.J.; Rebman, A.; Moon, K.A.; Aucott, J.; Heaney, C.; Schwartz, B.S. Obstacles to diagnosis and treatment of Lyme disease in the USA: A qualitative study. BMJ Open 2018, 8, e021367. [Google Scholar] [CrossRef]
- Johnson, L.; Aylward, A.; Stricker, R.B. Healthcare access and burden of care for patients with Lyme disease: A large United States survey. Health Policy 2011, 102, 64–71. [Google Scholar] [CrossRef]
- Johnson, L.; Wilcox, S.; Mankoff, J.; Stricker, R.B. Severity of chronic Lyme disease compared to other chronic conditions: A quality of life survey. PeerJ 2014, 2, e322. [Google Scholar] [CrossRef] [Green Version]
- Asch, E.S.; Bujak, D.I.; Weiss, M.; Peterson, M.G.; Weinstein, A. Lyme disease: An infectious and postinfectious syndrome. J. Rheumatol. 1994, 21, 454–461. [Google Scholar]
- Shadick, N.A.; Phillips, C.B.; Logigian, E.L.; Steere, A.C.; Kaplan, R.F.; Berardi, V.P.; Duray, P.H.; Larson, M.G.; Wright, E.A.; Ginsburg, K.S.; et al. The Long-Term Clinical Outcomes of Lyme Disease: A Population-Based Retrospective Cohort Study. Ann. Intern. Med. 1994, 121, 560–567. [Google Scholar] [CrossRef]
- Shadick, N.A.; Phillips, C.B.; Sangha, O.; Logigian, E.L.; Kaplan, R.F.; Wright, E.A.; Fossel, A.H.; Fossel, K.; Berardi, V.; Lew, R.A.; et al. Musculoskeletal and Neurologic Outcomes in Patients with Previously Treated Lyme Disease. Ann. Intern. Med. 1999, 131, 919–926. [Google Scholar] [CrossRef]
- Treib, J.; Fernandez, A.; Haass, A.; Grauer, M.T.; Holzer, G.; Woessner, R. Clinical and serologic follow-up in patients with neuroborreliosis. Neurology 1998, 51, 1489–1491. [Google Scholar] [CrossRef]
- Rebman, A.W.; Aucott, J.N. Post-treatment Lyme Disease as a Model for Persistent Symptoms in Lyme Disease. Front. Med. 2020, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Shor, S.; Green, C.; Szantyr, B.; Phillips, S.; Liegner, K.; Burrascano, J.J., Jr.; Bransfield, R.C.; Maloney, E.L. Chronic Lyme Disease: An Evidence-Based Definition by the ILADS Working Group. Antibiotics 2019, 8, 269. [Google Scholar] [CrossRef] [Green Version]
- Hook, S.A.; Nelson, C.A.; Mead, P.S. U.S. public’s experience with ticks and tick-borne diseases: Results from national HealthStyles surveys. Ticks Tick Borne Dis. 2015, 6, 483–488. [Google Scholar] [CrossRef]
- Johnson, L.; Shapiro, M.; Mankoff, J. Removing the Mask of Average Treatment Effects in Chronic Lyme Disease Research Using Big Data and Subgroup Analysis. Healthcare 2018, 6, 124. [Google Scholar] [CrossRef] [Green Version]
- Connor, S. Glaxo chief: Our drugs do not work on most patients. Independent 2003, 8, 1–2. [Google Scholar]
- Junghard, O.; Halling, K. Responsiveness of measures of heartburn improvement in non-erosive reflux disease. Health Qual. Life Outcomes 2007, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Kamper, S.J.; Maher, C.G.; Mackay, G. Global Rating of Change Scales: A Review of Strengths and Weaknesses and Considerations for Design. J. Man. Manip. Ther. 2009, 17, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Donta, S.T. Tetracycline therapy for chronic Lyme disease. Clin. Infect. Dis. 1997, 25 (Suppl. 1), S52–S56. [Google Scholar] [CrossRef]
- Donta, S.T. Macrolide therapy of chronic Lyme Disease. Med. Sci. Monit. 2003, 9, PI136–PI142. [Google Scholar]
- Scotti, J.M. Sex Differences in Lyme Disease Symptomatology in the Northeast United States. Ph.D. Thesis, Walden University, Minneapolis, MN, USA, 2020. [Google Scholar]
- Centers for Disease Control and Prevention. Measuring Healthy Days; CDC: Atlanta, GA, USA, 2000.
- DeSalvo, K.B.; Fan, V.S.; McDonell, M.B.; Fihn, S.D. Predicting Mortality and Healthcare Utilization with a Single Question. Health Serv. Res. 2005, 40, 1234–1246. [Google Scholar] [CrossRef] [Green Version]
- Fallon, B.A.; Keilp, J.G.; Corbera, K.M.; Petkova, E.; Britton, C.B.; Dwyer, E.; Slavov, I.; Cheng, J.; Dobkin, J.; Nelson, D.R.; et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology 2008, 70, 992–1003. [Google Scholar] [CrossRef] [Green Version]
- Fallon, B.A.; Zubcevik, N.; Bennett, C.; Doshi, S.; Rebman, A.W.; Kishon, R.; Moeller, J.R.; Octavien, N.R.; Aucott, J.N. The General Symptom Questionnaire-30 (GSQ-30): A Brief Measure of Multi-System Symptom Burden in Lyme Disease. Front. Med. 2019, 6, 283. [Google Scholar] [CrossRef] [Green Version]
- Krupp, L.B.; Hyman, L.G.; Grimson, R.; Coyle, P.K.; Melville, P.; Ahnn, S.; Dattwyler, R.; Chandler, B. Study and treatment of post Lyme disease (STOP-LD): A randomized double masked clinical trial. Neurology 2003, 60, 1923–1930. [Google Scholar] [CrossRef]
- Vendrow, J.; Haddock, J.; Needell, D.; Johnson, L. Feature Selection on Lyme Disease Patient Survey Data. arXiv 2020, arXiv:2009.09087. [Google Scholar]
- Centers for Disease Control and Prevention. Racial/Ethnic Disparities in Self-Rated Health Status Among Adults With and Without Disabilities—United States, 2004–2006. MMWR. Morb. Mortal. Wkly. Rep. 2008, 57, 1069. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.B.; Smalley, J.B. Engaging the Patient: Patient-Centered Research. In Strategies for Team Science Success: Handbook of Evidence-Based Principles for Cross-Disciplinary Science and Practical Lessons Learned from Health Researchers; Hall, K.L., Vogel, A.L., Croyle, R.T., Eds.; Springer: Cham, Switzerland, 2019; pp. 135–147. [Google Scholar]
- Agency for Healthcare Research and Quality. 21st Century Patient Registries, Ebook Addendum to Registries for Evaluating Patient Outcomes: A User’s Guide, 3rd ed.; Gliklich, R.E., Dreyer, N.A., Leavy, M.B., Christian, J.B., Eds.; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2018.
- Klempner, M.S.; Hu, L.T.; Evans, J.; Schmid, C.H.; Johnson, G.M.; Trevino, R.P.; Norton, D.; Levy, L.; Wall, D.; McCall, J.; et al. Two Controlled Trials of Antibiotic Treatment in Patients with Persistent Symptoms and a History of Lyme Disease. N. Engl. J. Med. 2001, 345, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Adrion, E.R.; Aucott, J.N.; Lemke, K.W.; Weiner, J.P. Health Care Costs, Utilization and Patterns of Care following Lyme Disease. PLoS ONE 2015, 10, e0116767. [Google Scholar] [CrossRef]
- FAIR Health. Trends and Patterns in Lyme Disease: An Analysis of Private Claims Data; White Paper; FAIR Health: New York, NY, USA, 2009. [Google Scholar]
- Zhang, X.; Meltzer, M.I.; Peña, C.A.; Hopkins, A.B.; Wroth, L.; Fix, A.D. Economic Impact of Lyme Disease. Emerg. Infect. Dis. 2006, 12, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Voqui, J. Clinical Outcome Assessment Implementation in Clinical Trials. In Proceedings of the Regulatory Education for Industry (REdi), Silver Spring, MD, USA, 29 September 2015; Available online: https://www.fda.gov/media/94051/download (accessed on 30 September 2020).
- Food and Drug Administration. Clinical Outcome Assessment (COA) Compendium; Food and Drug Administration: Silver Spring, MD, USA, 2009. Available online: https://www.fda.gov/media/130138/download (accessed on 30 September 2020).
- Johnson, L. Treatment Outcomes Important to Lyme Patients with Persistent Lyme Disease: A Chart Book. LymeDisease.org 2015. Available online: https://www.lymedisease.org/wp-content/uploads/2015/04/lymedisease.org-patient-survey-20151.pdf (accessed on 30 September 2020).
- Zhao, G.; Okoro, C.A.; Hsia, J.; Town, M. Self-Perceived Poor/Fair Health, Frequent Mental Distress, and Health Insurance Status Among Working-Aged US Adults. Prev. Chronic Dis. 2018, 15, 170523. [Google Scholar] [CrossRef] [Green Version]
- Stricker, R.B.; Johnson, L. Lyme disease: The next decade. Infect. Drug Resist. 2011, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Delong, A.K.; Blossom, B.; Maloney, E.L.; Phillips, S.E. Antibiotic retreatment of Lyme disease in patients with persistent symptoms: A biostatistical review of randomized, placebo-controlled, clinical trials. Contemp. Clin. Trials 2012, 33, 1132–1142. [Google Scholar] [CrossRef]
- Chandra, A.M.; Keilp, J.G.; Fallon, B.A. Correlates of perceived health-related quality of life in post-treatment Lyme encephalopathy. Psychosomatics 2013, 54, 552–559. [Google Scholar] [CrossRef] [Green Version]
- Fallon, B.A.; Petkova, E.; Keilp, J.G.; Britton, C.B. A Reappraisal of the U.S. Clinical Trials of Post-Treatment Lyme Disease Syndrome. Open Neurol. J. 2012, 6, 79–87. [Google Scholar] [CrossRef] [PubMed]
- National Academy of Medicine. Clinical Practice Guidelines We Can Trust; National Academies Press: Washington, DC, USA, 2011; p. 217. [Google Scholar]
- Cameron, D.J.; Johnson, L.B.; Maloney, E.L. Evidence assessments and guideline recommendations in Lyme disease: The clinical management of known tick bites, erythema migrans rashes and persistent disease. Expert Rev. Anti Infect. Ther. 2014, 12, 1103–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wormser, G.P.; Dattwyler, R.J.; Shapiro, E.D.; Halperin, J.J.; Steere, A.C.; Klempner, M.S.; Krause, P.J.; Bakken, J.S.; Strle, F.; Stanek, G.; et al. The Clinical Assessment, Treatment, and Prevention of Lyme Disease, Human Granulocytic Anaplasmosis, and Babesiosis: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2006, 43, 1089–1134. [Google Scholar] [CrossRef] [PubMed]
- Hook, S.; Nelson, C.; Mead, P. Self-reported Lyme disease diagnosis, treatment, and recovery: Results from 2009, 2011, & 2012 Health Styles nationwide surveys. In Proceedings of the 13th International Conference on Lyme Borreliosis and Other Tick Borne Diseases, Boston, MA, USA, 19 August 2013. [Google Scholar]
- Ziska, M.H.; Donta, S.T.; Demarest, F.C. Physician preferences in the diagnosis and treatment of Lyme disease in the United States. Infection 1996, 24, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Stricker, R.B.; Delong, A.K.; Green, C.L.; Savely, V.R.; Chamallas, S.N.; Johnson, L. Benefit of intravenous antibiotic therapy in patients referred for treatment of neurologic Lyme disease. Int. J. Gen. Med. 2011, 4, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Cameron, D. Results from Lyme disease treatment trial. In Proceedings of the Columbia University/LDA Conference, Lyme & Other Tick-Borne Diseases: Emerging Tick-Borne Diseases, Philadelphia, PA, USA, 28 October 2005. [Google Scholar]
- Small, P.M.; Fujiwara, P.I. Management of Tuberculosis in the United States. N. Engl. J. Med. 2001, 345, 189–200. [Google Scholar] [CrossRef]
- Bodur, H.; Erbay, A.; Colpan, A.; Akinci, E.; Akıncı, E. Brucellar spondylitis. Rheumatol. Int. 2003, 24, 221–226. [Google Scholar] [CrossRef]
- Garner, J.P.; Macdonald, M.; Kumar, P. Abdominal actinomycosis. Int. J. Surg. 2007, 5, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Freeman, H.J. Tropheryma whipplei infection. World J. Gastroenterol. 2009, 15, 2078–2080. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.H.; Wang, X.G.; Gao, J.S.; Qingyao, Y.; Horton, J. Continuous albendazole therapy in alveolar echinococcosis: Long-term follow-up observation of 20 cases. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 768–778. [Google Scholar] [CrossRef]
- Joynt, K.E.; Orav, E.J.; Jha, A.K. Physician Volume, Specialty, and Outcomes of Care for Patients With Heart Failure. Circ. Hear. Fail. 2013, 6, 890–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birkhäuer, J.; Gaab, J.; Kossowsky, J.; Hasler, S.; Krummenacher, P.; Werner, C.; Gerger, H. Trust in the health care professional and health outcome: A meta-analysis. PLoS ONE 2017, 12, e0170988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, L. Patient Preferences in Lyme Disease Treatment and Guidelines Chart Book. Figshare 2020. [Google Scholar] [CrossRef]
- Howie, L.; Hirsch, B.; Locklear, T.; Abernethy, A.P. Assessing The Value Of Patient-Generated Data To Comparative Effectiveness Research. Health Aff. 2014, 33, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, N.; Franklin, P.; Haynes, K. Direct-To-Patient Registry and Other Patient-Centric Designs. In 21st Century Patient Registries: Registries for Evaluating Patient Outcomes: A User’s Guide: 3rd Edition, Addendum [Internet]; Gliklich, R.E., Dreyer, N.A., Leavy, M.B., Eds.; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2018. [Google Scholar]
- Biau, D.J.; Kernéis, S.; Porcher, R. Statistics in Brief: The Importance of Sample Size in the Planning and Interpretation of Medical Research. Clin. Orthop. Relat. Res. 2008, 466, 2282–2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyatt, G.H.; Mills, E.J.; Elbourne, D. In the Era of Systematic Reviews, Does the Size of an Individual Trial Still Matter? PLoS Med. 2008, 5, e4. [Google Scholar] [CrossRef] [Green Version]
- National Academies of Sciences, Engineering, and Medicine. Enabling Precision Medicine: The Role of Genetics in Clinical Drug Development: Proceedings of a Workshop; The National Academies Press: Washington, DC, USA, 2017. [Google Scholar]
- Saver, J.L.; Kalafut, M. Combination Therapies and the Theoretical Limits of Evidence-Based Medicine. Neuroepidemiology 2001, 20, 57–64. [Google Scholar] [CrossRef]
- Kent, D.M.; Steyerberg, E.; Van Klaveren, D. Personalized evidence based medicine: Predictive approaches to heterogeneous treatment effects. BMJ 2018, 363, k4245. [Google Scholar] [CrossRef]
- Cohen, D.J.; Keller, S.R.; Hayes, G.R.; Dorr, D.A.; Ash, J.S.; Sittig, D.F.; Post, L.; Rosenbloom, T. Integrating Patient-Generated Health Data Into Clinical Care Settings or Clinical Decision-Making: Lessons Learned From Project HealthDesign. JMIR Hum. Factors 2016, 3, e26. [Google Scholar] [CrossRef]
- Wood, W.A.; Bennett, A.V.; Basch, E. Emerging uses of patient generated health data in clinical research. Mol. Oncol. 2015, 9, 1018–1024. [Google Scholar] [CrossRef]
- Bayliss, M.; Rendas-Baum, R.; White, M.K.; Maruish, M.; Bjorner, J.B.; Tunis, S.L. Health-related quality of life (HRQL) for individuals with self-reported chronic physical and/or mental health conditions: Panel survey of an adult sample in the United States. Health Qual. Life Outcomes 2012, 10, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bytzer, P. Assessment of reflux symptom severity: Methodological options and their attributes. Gut 2004, 53, iv28–iv34. [Google Scholar] [CrossRef] [PubMed]
- Citera, M.; Freeman, P.R.; Horowitz, R.I. Empirical Validation of the Horowitz Multiple Systemic Infectious Disease Syndrome Questionnaire for Suspected Lyme Disease. Int. J. Gen. Med. 2017, 10, 249–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stricker, R.B.; Johnson, L. Gender Bias in Chronic Lyme Disease. J. Womens Health 2009, 18, 1717–1718. [Google Scholar] [CrossRef] [PubMed]
- Kent, D.M.; Hayward, R.A. Limitations of Applying Summary Results of Clinical Trials to Individual Patients: The need for risk stratification. JAMA 2007, 298, 1209–1212. [Google Scholar] [CrossRef]




| Variable | Unwell % | Non Responder | Low Responder | High Responder | Well |
|---|---|---|---|---|---|
| Chronic Lyme disease a | 100% | 100% | 100% | 100% | 100% |
| Stage When Diagnosed | |||||
| Late untreated Lyme disease b | 77% | 76% | 78% | 78% | 66% |
| Early Lyme disease c | 17% | 18% | 16% | 17% | 29% |
| Don’t know/Other | 6% | 6% | 7% | 5% | 4% |
| Key Diagnostic Factors | |||||
| Clinician diagnosed d | 100% | 100% | 100% | 100% | 100% |
| Recollection of tick bite | 43% | 44% | 45% | 42% | 40% |
| Recollection of EM rash e | 35% | 34% | 37% | 36% | 30% |
| With supportive lab tests | 80% | 81% | 77% | 79% | 79% |
| 1 or more coinfection | 70% | 62% | 69% | 78% | 63% |
| Disabled (with or without disability benefits) | 35% | 41% | 38% | 26% | NA |
| Variable | Count (% of CLD Well and Unwell) |
|---|---|
| Gender a | |
| Female | 1943 (85%) |
| Mean age | 49 |
| Education b | |
| High school or less | 177 (8%) |
| Some college or associate degree | 727 (33%) |
| Bachelor degree | 695 (32%) |
| Graduate school degree | 577 (27%) |
| Family income c | |
| Less than $25 k | 295 (17%) |
| $25–50 k | 308 (17%) |
| $50–75 k | 322 (18%) |
| $75–100 k | 227 (13%) |
| >$100 k | 631 (35%) |
| Geography d | |
| East | 594 (26%) |
| Midwest | 317 (14%) |
| South | 624 (27%) |
| West | 737 (32%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, L.; Shapiro, M.; Stricker, R.B.; Vendrow, J.; Haddock, J.; Needell, D. Antibiotic Treatment Response in Chronic Lyme Disease: Why Do Some Patients Improve While Others Do Not? Healthcare 2020, 8, 383. https://doi.org/10.3390/healthcare8040383
Johnson L, Shapiro M, Stricker RB, Vendrow J, Haddock J, Needell D. Antibiotic Treatment Response in Chronic Lyme Disease: Why Do Some Patients Improve While Others Do Not? Healthcare. 2020; 8(4):383. https://doi.org/10.3390/healthcare8040383
Chicago/Turabian StyleJohnson, Lorraine, Mira Shapiro, Raphael B. Stricker, Joshua Vendrow, Jamie Haddock, and Deanna Needell. 2020. "Antibiotic Treatment Response in Chronic Lyme Disease: Why Do Some Patients Improve While Others Do Not?" Healthcare 8, no. 4: 383. https://doi.org/10.3390/healthcare8040383
APA StyleJohnson, L., Shapiro, M., Stricker, R. B., Vendrow, J., Haddock, J., & Needell, D. (2020). Antibiotic Treatment Response in Chronic Lyme Disease: Why Do Some Patients Improve While Others Do Not? Healthcare, 8(4), 383. https://doi.org/10.3390/healthcare8040383






